
Author: Emma Thomée*, PhD candidate
Email: emma.thomee@elvesys.com
1. Introduction into lung-on-a-chip microfluidic systems
Organ-on-chip technology provides unique opportunities to study lung physiology and pathophysiology in vitro. The first lung-on-a-chip platforms emerged almost 10 years ago, and significant progress has since been made in terms of both biological and engineering complexity. Here, we describe one of the most sophisticated lung-on-a-chip systems in terms of biological complexity [1] that model the alveolar-capillary barrier, in order to present the current knowledge on the topic.
2. Structure and function of the human lung
Organ-on-chip technology provides unique opportunities to study lung physiology and pathophysiology in vitro. The first lung-on-a-chip platforms emerged almost 10 years ago, and significant progress has since been made in terms of both biological and engineering complexity. Here, we describe one of the most sophisticated lung-on-a-chip systems in terms of biological complexity [1] that model the alveolar-capillary barrier, in order to present the current knowledge on the topic.
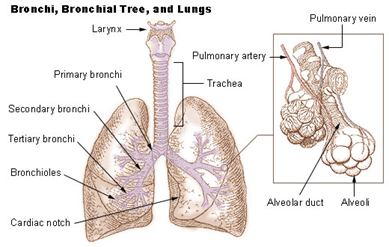
Figure 1. Illustration of the tree lung structure including the components: trachea, bronchi and bronchioles. The magnification is showing alveoli surrounded by a mesh of capillaries – where the gas exchange occurs. (reprinted from https://training.seer.cancer.gov/anatomy/respiratory/
passages/bronchi.html)
3. In-vitro models of the alveolar-capillary barrier
The alveolar barrier thus plays a vital role allowing oxygen to enter the body, and failure of the alveoli can be a very serious condition. Infection diseases in the alveoli, such as pulmonary edema or pneumonia affects hundreds of millions of people globally and results in millions of deaths every year. Additionally, oral inhalation of drugs is a common mean of drug delivery, and understanding what substances can pass across the alveolar barrier is therefore of great importance. As in most fields of biomedical research, animal models are commonly used to study lung pathology and for developing new treatments. Ethical issues limit the use of animal models for research and furthermore animal lungs are substantially different from human lungs [2], therefore limiting the translation of results. This has motivated the development of in vitro platforms for lung research. Read more about in vitro platforms for lung research here.
4. Organ-on-a-chip technology to model the alveolar-capillary barrier
The term “organ-on-a-chip” as well as the technology was developed by Donald Ingber, founder of the Wyss Institute at Harvard University. Organ-on-a-chip systems are essentially micro-engineered devices containing living cells, that mimic functions of human organs. The team of Donald Ingber developed the seminal breathing lung-on-a-chip model that incorporated the mechanical cues of breathing in organ-on-chip devices. Following the first publication of this model, the team has published several studies to model different pathological conditions of respiratory diseases on chip [3, 4, 5, 6].
A comprehensive review about organ-on-a-chip technology can be found here.
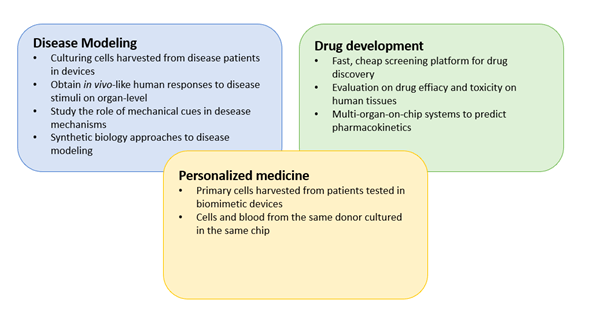
5. Lung-on-a-chip architecture
Read about the engineering aspects of lung-on-a-chip devices here .
Compared to the previous generation of lung-on-a-chip models, the most recent models developed by the Ingber group include the following novel features:
The use of human primary cells
Primary human lung alveolar epithelial cells and human vascular endothelial cells were seeded in the mode, instead of cancer cell line. This is of significant importance as primary cells provide the opportunity to link the results to the donor they were acquired from – an important step towards personalized medicine. Furthermore, primary cells more truly replicate the diversity of human tissue compared to cell lines which have developed homogeneous phenotypes as a result of passaging.
Microfluidic perfusion of whole blood
All four walls of the endothelial compartment of the chip was lined with a continuous layer of endothelial cells, forming a “vascular tube”. This enabled perfusion of whole blood through the lumen of the vascular tube without thrombus formation as it normally occurs if blood is perfused through a microchannel, even when coated with extracellular matrix proteins.
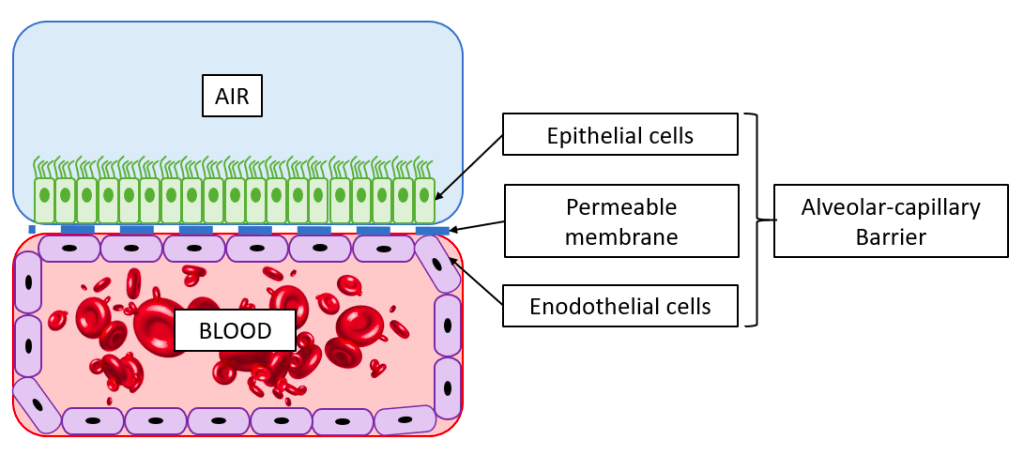
6. Disease modelling in microfluidic lung-on-a-chip systems
Mimicking pathological conditions in organ-on-a-chip models that accurately represent human physiology is of great interest for future drug development, as well as for fundamental research into respiratory diseases. This culture model enabled experimental investigation of intravascular thrombosis under hemodynamic conditions. The disease state was induced by perfusing tumor necrosis factor-α (TNF-α), an inflammatory stimulant, in the fluid compartment. This resulted in disease features such as platelet aggregation dose-dependent and inflammation, as also accrues in patients exposed to TNF-α. Furthermore, this system highlighted one of the main advantages of organ-on-a-chip systems for disease modelling: the ability to study the contribution of individual tissue components of the organ compared to their cooperative responses at the organ-level. This type of synthetic biology approach is naturally not seen in animal studies, where only system level responses can be obtained
7. Future directions of lung-on-a-chip development
Although proved to be a promising technique for future lung in-vitro research, further research is needed to develop more physiologically relevant and practical systems. The biological component of lung-on-chip devices are still oversimplified and lack cellular components such as immune cell or neural cells. The study presented in this review demonstrated significant improvements by using primary cells and perfusing whole blood in the system. Furthermore, the engineering aspects such as device material, sensor integration and compatibility with other laboratory equipment are expected to progress in the coming years.
To learn more about the considerations building an air-liquid interface for a microfluidic lung-on-a-chip model, look here.
References
[1] Jain, A., Barrile, R., van der Meer, A., Mammoto, A., Mammoto, T., De Ceunynck, K., Aisiku, O., Otieno, M., Louden, C., Hamilton, G., Flaumenhaft, R., Ingber, D., 2018. Primary Human Lung Alveolus-on-a-chip Model of Intravascular Thrombosis for Assessment of Therapeutics. Clin. Pharmacol. Ther. 103, 332–340. https://doi.org/10.1002/cpt.742
[2] Miller, A.J., Spence, J.R., 2017. In Vitro Models to Study Human Lung Development, Disease and Homeostasis. Physiology 32, 246–260. https://doi.org/10.1152/physiol.00041.2016
[3] Benam, Kambez H., Novak, R., Nawroth, J., Hirano-Kobayashi, M., Ferrante, T.C., Choe, Y., Prantil-Baun, R., Weaver, J.C., Bahinski, A., Parker, K.K., Ingber, D.E., 2016. Matched-Comparative Modeling of Normal and Diseased Human Airway Responses Using a Microengineered Breathing Lung Chip. Cell Syst. 3, 456-466.e4. https://doi.org/10.1016/j.cels.2016.10.003
[4] Benam, Kambez H, Villenave, R., Lucchesi, C., Varone, A., Hubeau, C., Lee, H.-H., Alves, S.E., Salmon, M., Ferrante, T.C., Weaver, J.C., Bahinski, A., Hamilton, G.A., Ingber, D.E., 2016. Small airway-on-a-chip enables analysis of human lung inflammation and drug responses in vitro. Nat. Methods 13, 151–157. https://doi.org/10.1038/nmeth.3697
[5] Hassell, B.A., Goyal, G., Lee, E., Sontheimer-Phelps, A., Levy, O., Chen, C.S., Ingber, D.E., 2017. Human organ chip models recapitulate orthotopic lung cancer growth, therapeutic responses, and tumor dormancy in vitro. Cell Rep. 21, 508–516.
[6] Huh, D., Leslie, D.C., Matthews, B.D., Fraser, J.P., Jurek, S., Hamilton, G.A., Thorneloe, K.S., McAlexander, M.A., Ingber, D.E., 2012. A human disease model of drug toxicity-induced pulmonary edema in a lung-on-a-chip microdevice. Sci. Transl. Med. 4. https://doi.org/10.1126/scitranslmed.3004249
Source of original article: https://www.elveflow.com/microfluidic-reviews/organs-on-chip-3d-cell-culture/overview-lung-on-chip-technology-use-of-alveolar-capillary-barrier-human-medicine/